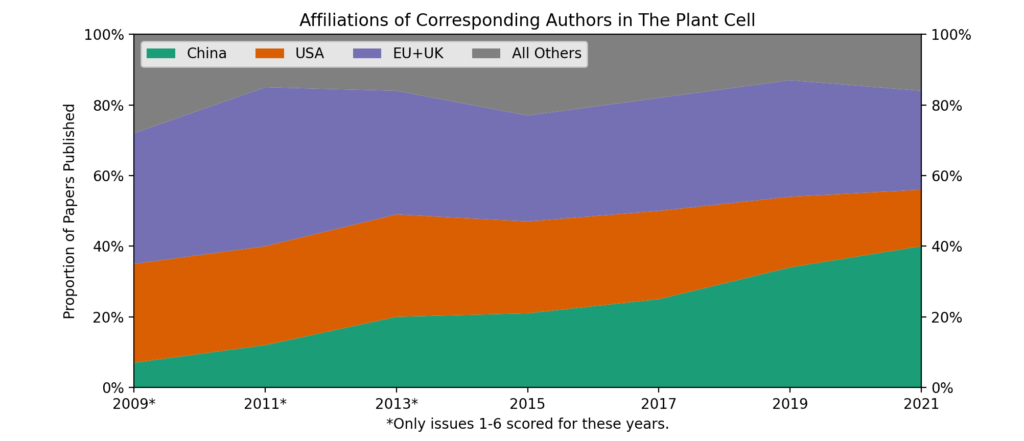
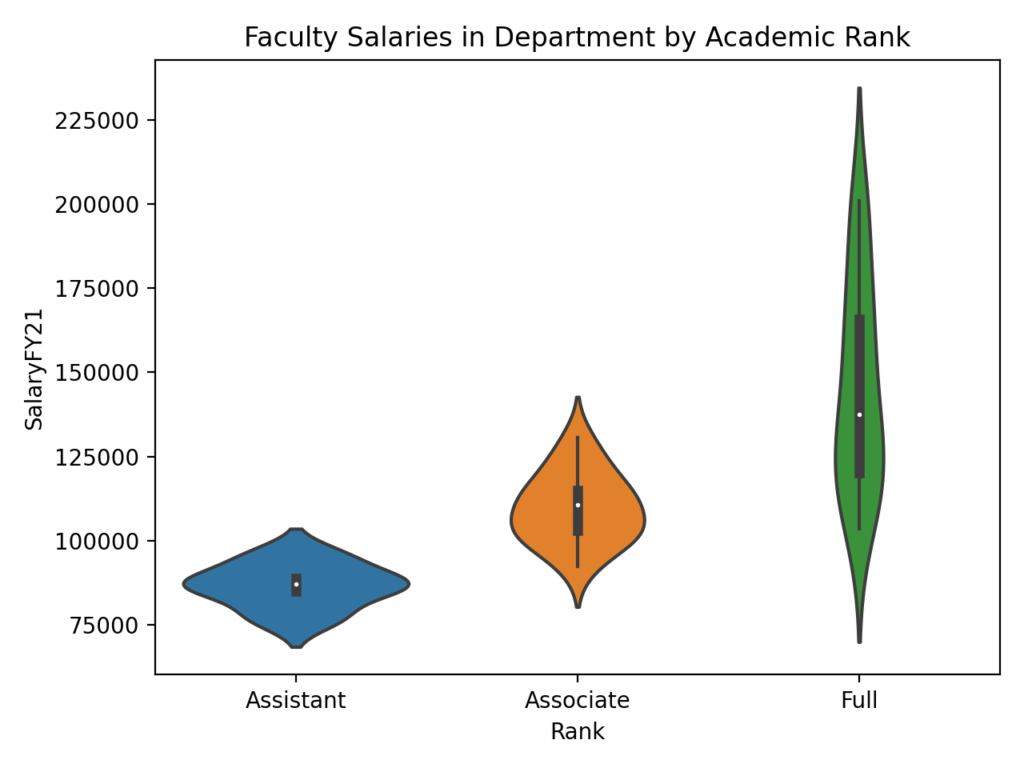
Lightly modified from my pitch to the university’s news/public relations office.
My collaborator Katarzyna Glowacka’s lab studies a process called non-photochemical quenching, which essentially acts as a surge protector for photosynthesis. If the amount of light hitting a plant’s leaf suddenly increases, for example if a cloud moves off the sun or wind blows a leaf from shadow into direct sunlight, non-photochemical quenching helps damp down the sudden surge of light energy that could otherwise damage or destroy the cell. Work Prof. Glowacka did as a postdoc showed that using transgenic technology it is possible to improve how fast non-photochemical quenching turns off and on and doing this can increase both photosynthetic productivity and overall crop yield.
Today, a new study came out in New Phytologist that was the result of a 3.5 year collaboration between my lab and Prof. Glowacka’s. Over two field season’s members of the Glowacka lab used a new high throughput technique to measure non-photochemical quenching in more than 700 types of corn grown in replicated field trials. A former postdoc in my lab, now on the faculty at the University of Warsaw, Marcin Grzybowski, used the measurements from the Glowacka lab to identify genes in the corn genome that controlled naturally occurring variation in the speed of non-photochemical quenching. The Glowacka lab was then able to confirm that these genes really do help regulate the process by looking at mutations of the same genes in arabidopsis (a model plant that is faster to work with than corn).
The big potential implications of this study are:
- Discovering new genes regulating non-photochemical quenching which no one knew were involved before. The most important/exciting of these is gene called PSI3.
- Showing that there is a lot of naturally occurring variation in non-photochemical quenching in corn already, so it wouldn’t require a 10-15 year and $100M+ effort to translate her postdoctoral research into higher yielding corn varieties for Nebraska farmers, it may be possible to achieve the same outcome with conventional marker assisted breeding in ½ the time and at less than 5% of the cost of commercializing a new transgenic.
Seema Sahay, Marcin Grzybowski, James C. Schnable, Katarzyna Głowacka (2023) “Genetic control of photoprotection and photosystem II operating efficiency in plants.” New Phytologist doi: 10.1111/nph.18980